Subcutaneous Ticks: An Overview
What are Subcutaneous Ticks?
Distinguishing Features
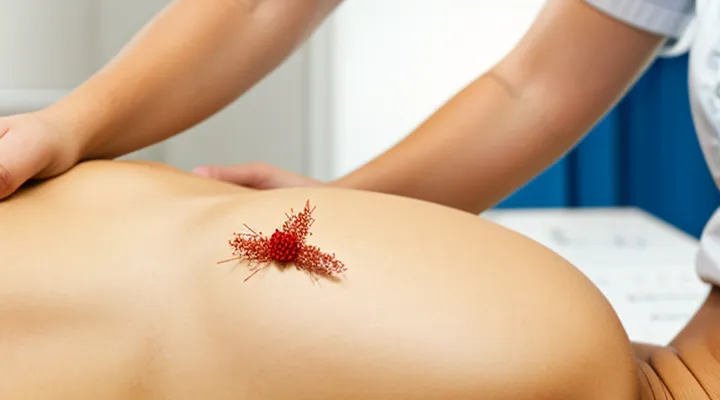
Subcutaneous ticks embed beneath the epidermis, creating a distinct clinical picture that differs from superficial attachment. The following characteristics enable reliable identification:
- Localized swelling: A firm, often circular nodule forms around the tick’s mouthparts, typically 0.5–2 cm in diameter.
- Skin discoloration: The overlying epidermis may appear erythematous or violaceous; a central punctum, sometimes invisible, marks the insertion site.
- Absence of movement: Unlike crawling ticks, the lesion remains static; patients report no sensation of a live organism moving under the skin.
- Pain profile: Mild to moderate discomfort is common, frequently described as a deep ache rather than surface irritation.
- Delayed itching: Pruritus usually develops days after the bite, contrasting with immediate itching of superficial ticks.
- Resistance to removal: Standard tweezers cannot extract the organism; removal often requires a small incision or surgical extraction.
- Ultrasound visibility: High‑frequency ultrasound reveals a hypoechoic, oval structure within the dermis or subcutaneous tissue, confirming presence.
Recognition of these features facilitates prompt diagnosis and appropriate treatment, reducing the risk of secondary infection or pathogen transmission.
Common Misconceptions
Subcutaneous ticks are arachnids that penetrate the epidermis and lodge their mouthparts within the dermal layer, often remaining hidden for days or weeks. Their presence can be mistaken for other skin conditions, leading to persistent myths.
- Misconception: The tick attaches only to the surface of the skin. Reality: After initial attachment, the feeding apparatus can migrate deeper, creating a pocket beneath the epidermis that is not visible without close inspection or imaging.
- Misconception: All embedded ticks cause immediate pain or itching. Reality: Many subdermal ticks release anesthetic compounds, producing no sensation until they detach or become inflamed.
- Misconception: Removing the tick with tweezers eliminates the problem. Reality: Surface removal may leave mouthparts embedded, which can continue feeding and provoke local inflammation or infection.
- Misconception: Tick‑borne diseases require a visible bite. Reality: Pathogens can be transmitted through the saliva injected during feeding, even when the tick remains unseen beneath the skin.
- Misconception: Only outdoor exposure leads to subcutaneous ticks. Reality: Ticks can be transported on clothing or pets into indoor environments, where they may attach unnoticed during brief contact.
Accurate identification involves visual examination for a small, raised nodule or a dark spot indicating the tick’s body, sometimes aided by dermatoscopy. If a subdermal tick is suspected, medical extraction under sterile conditions is recommended to minimize tissue damage and reduce infection risk.
Pathophysiology of Infestation
Life Cycle and Transmission
Stages of Development
Subcutaneous ticks become embedded in human tissue through a series of developmental phases that follow the insect’s normal life cycle. The process begins when a questing tick, usually a larva or nymph, attaches to the skin and inserts its hypostome deep enough to breach the epidermis. Salivary secretions contain enzymes that facilitate tissue penetration and suppress local immune responses, allowing the tick to migrate beneath the dermal layer.
Once beneath the surface, the tick enters the feeding stage. It remains attached for several days, expanding as it ingests blood. During this period, the tick’s body enlarges markedly, producing a palpable nodule that may be mistaken for a cyst or lipoma. The feeding phase is divided into three sub‑stages:
- Early engorgement – the tick’s abdomen swells to approximately 1–2 mm; blood intake is modest, and the host may experience mild itching.
- Mid‑engorgement – the abdomen reaches 3–5 mm; blood consumption accelerates, and the surrounding tissue may become inflamed or tender.
- Late engorgement – the abdomen expands to 6–10 mm; the tick approaches maximal weight, and the host often notices a firm, raised lump.
After reaching full engorgement, the tick detaches or is expelled, sometimes leaving a residual cavity that can become infected if not treated. In the case of adult females that remain attached, the next developmental step is oviposition, although this rarely occurs on human hosts. The entire sequence, from attachment to detachment, typically spans 4–10 days, depending on tick species, life stage, and host immune response.
Modes of Transfer
Ticks can become embedded beneath the skin through several distinct mechanisms. The process does not require a deliberate act by the parasite; rather, it results from the interaction between tick behavior, host skin characteristics, and environmental conditions.
- Direct attachment and prolonged feeding – Certain species, such as Dermacentor and Ixodes, insert their mouthparts deep into the epidermis. Extended attachment time increases the likelihood that the hypostome penetrates the dermal layer, leading to subcutaneous positioning.
- Host‑induced migration – After initial attachment, the tick may migrate along the skin surface, seeking a more secure site. In some cases, movement pushes the feeding apparatus into deeper tissue, especially on areas with thin stratum corneum.
- Skin trauma and micro‑abrasions – Minor cuts, scrapes, or punctures created by clothing, equipment, or outdoor activities provide entry points. Ticks exploit these openings, bypassing the outer epidermal barrier and entering the subdermal space.
- Self‑inoculation during grooming – When a host removes a partially attached tick, forceful pulling can rupture the tick’s body, depositing its mouthparts and internal contents beneath the skin. This accidental inoculation often results in a localized subcutaneous nodule.
- Environmental pressure – High humidity and temperature accelerate tick activity, prompting more aggressive attachment attempts. Under such conditions, ticks may embed deeper to secure a stable feeding site.
Understanding these pathways clarifies why subdermal tick lesions appear after outdoor exposure, especially in regions where tick density and host‑contact rates are elevated.
Host-Parasite Interaction
Initial Penetration
Ticks attach to a host when they sense heat, carbon dioxide, and movement. The front legs, equipped with sensory organs, locate a suitable site—typically thin, hair‑free skin. The tick then inserts its hypostome, a barbed feeding tube, into the epidermis. Salivary secretions containing anticoagulants, anesthetics, and enzymes facilitate penetration by preventing clotting, dulling pain, and breaking down extracellular matrix proteins. Within seconds to minutes, the hypostome anchors firmly, allowing the tick to advance deeper into the dermis. Mechanical pressure from the barbs, combined with enzymatic digestion of tissue, creates a tunnel that houses the tick’s body beneath the skin surface. This initial breach establishes the subcutaneous position from which the parasite feeds for days to weeks.
Immune Response and Symptoms
When a tick penetrates the skin and settles beneath the epidermis, the innate immune system detects foreign proteins in the saliva. Mast cells release histamine, producing localized swelling and redness within minutes to hours. Neutrophils arrive at the site, attempting to phagocytose tick antigens, which often results in a palpable, tender nodule that can persist for several days.
Adaptive immunity develops as antigen‑presenting cells process tick proteins and stimulate T‑lymphocytes. Cytokine release amplifies inflammation, leading to erythema, warmth, and occasional pruritus. In some individuals, a delayed‑type hypersensitivity reaction emerges 48–72 hours after attachment, characterized by a larger, indurated lesion and intensified itching.
Common clinical manifestations include:
- Small, firm bump at the bite location
- Red halo surrounding the nodule
- Itching or burning sensation
- Mild fever or malaise if systemic exposure to tick‑borne pathogens occurs
- Rarely, regional lymphadenopathy indicating immune activation beyond the bite site
If the tick remains attached, prolonged exposure to its saliva can suppress local immune responses, allowing the parasite to feed undisturbed. This suppression may reduce visible inflammation but increases the risk of pathogen transmission. Prompt removal typically halts further immune modulation and limits lesion progression.
Common Types of Subcutaneous Ticks
Scabies Mites («Sarcoptes scabiei»)
Clinical Manifestations
Subcutaneous tick infestations produce distinct cutaneous findings that differ from superficial attachment. The tick’s mouthparts remain embedded beneath the dermis, generating a localized inflammatory response. Clinically, patients present with a palpable, firm nodule at the site of insertion. The nodule often measures 0.5–2 cm in diameter, may be tender or painless, and can develop a central punctum or ulceration as the tick migrates or dies. Overlying skin may display erythema, induration, or a violaceous hue.
Associated symptoms include pruritus, burning sensation, or a throbbing pain that intensifies with movement of the limb. In some cases, regional lymphadenopathy accompanies the lesion, reflecting a systemic immune reaction. Systemic manifestations can arise when the tick transmits pathogens; fever, chills, malaise, headache, and myalgia may appear within days to weeks after infestation. Allergic responses range from localized urticaria to generalized urticaria, and, rarely, anaphylaxis.
Typical clinical picture:
- Firm subdermal nodule, sometimes with central punctum
- Erythema or discoloration of overlying skin
- Pain, pruritus, or burning sensation
- Regional lymph node enlargement
- Secondary infection signs: purulent discharge, increasing warmth
- Systemic signs: fever, chills, malaise, headache, myalgia
- Allergic reactions: urticaria, angioedema, anaphylaxis
Recognition of these signs enables timely removal of the tick and appropriate management of potential infectious or allergic complications.
Diagnosis and Differential Diagnosis
Subcutaneous tick infestation manifests as a small, often painless nodule beneath the skin, sometimes with a central punctum or a visible tick body. The overlying skin may appear erythematous or slightly raised, and patients may report a sensation of movement or itching. In some cases, the tick remains hidden, and only a localized inflammatory reaction is evident.
Accurate diagnosis requires a systematic approach. First, perform a thorough physical examination, noting the size, shape, and mobility of the lesion. Palpation may reveal a firm, elongated structure. Second, use dermoscopy or high‑resolution ultrasound to visualize the tick’s morphology and confirm its subdermal location. Third, consider removal of the specimen for direct identification under magnification, comparing morphological features (e.g., capitulum, legs, scutum) with taxonomic keys. Fourth, obtain a detailed exposure history, including recent outdoor activities, geographic region, and duration of attachment, to assess risk of pathogen transmission.
Differential diagnosis includes:
- Erythema migrans – expanding erythematous rash associated with early Lyme disease, lacking a palpable organism.
- Dermatofibroma – firm, dermal nodule without central punctum or movement.
- Insect bite reaction – superficial papule or vesicle, usually with acute pruritus and no embedded arthropod.
- Cutaneous cyst (epidermoid or pilomatricoma) – encapsulated lesion without external opening.
- Foreign‑body granuloma – inflammatory nodule surrounding a non‑biological material, often with a history of trauma.
- Bacterial abscess – tender, fluctuant collection, typically accompanied by signs of infection.
Laboratory testing is reserved for cases with systemic symptoms or suspicion of tick‑borne disease. Serologic assays for Borrelia burgdorferi, Anaplasma, or Rickettsia should be ordered based on regional prevalence and clinical presentation. Prompt removal of the tick, followed by appropriate antimicrobial prophylaxis when indicated, reduces the likelihood of complications.
Demodex Mites («Demodex folliculorum» and «Demodex brevis»)
Associated Conditions
Subcutaneous tick embedment occurs when a tick inserts its mouthparts into the dermis and remains beneath the epidermal layer, creating a concealed feeding site. This location can facilitate the transmission of a range of pathogens and provoke local tissue reactions, leading to distinct medical conditions.
- Lyme disease – spirochetal infection transmitted by Ixodes species; early signs include erythema migrans, followed by arthritis, neurologic deficits, and cardiac involvement if untreated.
- Anaplasmosis – caused by Anaplasma phagocytophilum; symptoms comprise fever, leukopenia, thrombocytopenia, and elevated liver enzymes.
- Babesiosis – protozoan infection (Babesia spp.) resulting in hemolytic anemia, hemoglobinuria, and possible organ failure in immunocompromised patients.
- Rocky Mountain spotted fever – Rickettsia rickettsii infection presenting with fever, rash, and vasculitis; delayed therapy increases mortality risk.
- Tick paralysis – neurotoxic protein secreted by certain tick species; progressive weakness that can culminate in respiratory failure, reversible upon tick removal.
- Bacterial superinfection – secondary infection of the bite site by Staphylococcus or Streptococcus species; manifests as cellulitis, abscess formation, or necrotizing fasciitis.
- Allergic reactions – localized urticaria, edema, or systemic anaphylaxis triggered by tick saliva proteins.
- Granuloma formation – chronic inflammatory response encapsulating tick remnants, producing palpable nodules that may persist for months.
- Necrosis – tissue death caused by prolonged ischemia or toxin release, leading to ulceration and potential need for surgical debridement.
Recognition of these conditions requires prompt clinical assessment and, when indicated, targeted antimicrobial or supportive therapy to prevent complications.
Factors Influencing Proliferation
Subcutaneous tick colonization results from a combination of biological, environmental, and host‑related variables that promote the parasite’s ability to embed beneath the skin and multiply.
Key determinants include:
- Species‑specific feeding behavior – Certain ixodid species possess mouthparts adapted for deep tissue penetration, enabling prolonged attachment and larval development.
- Ambient temperature and humidity – Warm, moist conditions accelerate tick metabolism, shorten the engorgement period, and increase survival rates of eggs deposited in the host’s dermal layer.
- Host skin integrity – Microabrasions, dermatological disorders, or compromised barrier function provide entry points for ticks to bypass the epidermis.
- Immune response modulation – Suppressed or altered host immunity, whether due to disease, medication, or genetic factors, reduces the effectiveness of inflammatory defenses that would otherwise expel the parasite.
- Blood‑feeding frequency – Repeated exposure to tick‑infested environments raises the probability of multiple bites, facilitating cumulative subcutaneous infestations.
Understanding these factors allows clinicians to anticipate risk scenarios, implement targeted preventive measures, and optimize treatment protocols for patients presenting with embedded ticks.
Risk Factors and Prevention
Environmental Factors
Areas of High Prevalence
Subcutaneous tick infestations are reported most frequently in regions where tick vectors thrive and human exposure is common. The distribution aligns with the habitats of Ixodes ricinus, Dermacentor variabilis, and Amblyomma americanum, which are capable of penetrating the skin and embedding their mouthparts beneath the epidermis.
- Northeastern United States (e.g., New York, Pennsylvania, Massachusetts): dense deciduous forests and high deer populations create optimal conditions for Ixodes scapularis.
- Upper Midwest (e.g., Wisconsin, Minnesota, Michigan): extensive grasslands and woodland edges support Dermacentor and Amblyomma species.
- Pacific Northwest (e.g., Washington, Oregon): temperate rainforests harbor Ixodes pacificus, a vector for subcutaneous attachment.
- Central and Southern Europe (e.g., Germany, Italy, Spain): mixed woodland and agricultural landscapes sustain Ixodes ricinus populations.
- Southeast Asia (e.g., Thailand, Malaysia): tropical climates and livestock farming promote Amblyomma and Haemaphysalis species capable of deeper skin penetration.
These areas share common ecological factors: abundant wildlife hosts, humid microclimates, and seasonal activity peaks that increase human‑tick contact. Awareness of regional risk patterns enables targeted prevention and early clinical recognition of subdermal tick lesions.
Occupational Hazards
Subcutaneous ticks embed their mouthparts into the dermal layer, releasing saliva that contains anticoagulants and immunomodulatory compounds. The feeding process can last several days, during which the arthropod remains concealed beneath the skin, often escaping visual detection until swelling or a palpable nodule develops.
Workers who routinely encounter vegetation, animal hosts, or natural habitats face the greatest exposure. Forestry crews, agricultural laborers, wildlife rehabilitators, and outdoor pest‑control technicians encounter tick‑infested environments more frequently than office‑based personnel. The combination of prolonged outdoor activity, limited protective barriers, and frequent contact with wildlife increases the probability of a tick achieving subdermal insertion.
Key exposure factors include:
- Dense underbrush or leaf litter that shelters questing ticks.
- Inadequate clothing coverage, such as short sleeves or open footwear.
- Absence of routine body inspections during and after work shifts.
- Use of equipment that forces prolonged contact with ground flora (e.g., ladders, pruning tools).
Preventive actions:
- Wear long‑sleeved, tightly woven garments and gaiters; treat fabrics with permethrin when permissible.
- Apply EPA‑registered repellents containing DEET, picaridin, or IR3535 to exposed skin before entering tick habitats.
- Conduct systematic tick checks at least every two hours and immediately after leaving the work area, focusing on scalp, armpits, groin, and interdigital spaces.
- Maintain cleared work zones by removing leaf litter and trimming low vegetation around occupational sites.
- Provide training on tick identification, attachment signs, and proper removal techniques.
When a subcutaneous tick is suspected, medical evaluation should occur promptly. Diagnosis relies on visual or palpation evidence of a localized swelling, often accompanied by erythema. Removal requires a sterile incision and extraction of the entire organism to prevent retained mouthparts. Post‑removal monitoring for erythema migrans, fever, or joint pain is essential, as early antimicrobial therapy can mitigate vector‑borne infections such as Lyme disease or rickettsial illnesses.
Personal Hygiene and Practices
Importance of Cleanliness
Subcutaneous tick attachment occurs when a tick penetrates the epidermis and embeds its mouthparts beneath the skin surface, often unnoticed until swelling or a localized lesion develops. The parasite’s saliva, introduced during feeding, may transmit pathogens and provoke inflammatory responses that complicate diagnosis and treatment.
Maintaining personal hygiene directly lowers the probability of such hidden infestations. Regular bathing removes detached arthropods before they can re‑attach, while thorough skin inspection after outdoor exposure reveals early-stage attachments. Key practices include:
- Daily washing of exposed areas with soap and water.
- Post‑activity visual examination of the entire body, focusing on scalp, armpits, groin, and interdigital spaces.
- Prompt removal of any attached tick using fine‑pointed tweezers, grasping close to the skin and pulling steadily to avoid mouthpart fragmentation.
Environmental cleanliness further supports prevention. Keeping lawns trimmed, removing leaf litter, and treating vegetation with acaricides diminish tick habitats, reducing the likelihood of contact. Wearing long sleeves and pants treated with permethrin adds a physical barrier that complements personal hygiene measures.
Protective Measures
Subcutaneous tick attachment occurs when a tick penetrates the skin and remains beneath the epidermis, often unnoticed until swelling or a lesion develops. Prevention relies on reducing exposure, creating barriers, and responding promptly to bites.
- Wear tightly woven, long‑sleeved shirts and full‑length trousers; tuck shirts into pants and pants into socks.
- Apply EPA‑registered repellents containing DEET, picaridin, or IR3535 to exposed skin and clothing.
- Treat outdoor gear, boots, and backpacks with permethrin; reapply after washing.
- Conduct full‑body inspections after leaving wooded or grassy areas, focusing on scalp, armpits, and groin.
Environmental control and behavioral precautions further limit risk.
- Maintain lawns by mowing regularly and removing leaf litter; create a clear perimeter around residential structures.
- Use acaricide treatments on high‑risk zones such as pet bedding and garden borders.
- Limit outdoor activity during peak tick activity periods (dawn and dusk) and avoid dense underbrush.
If a tick is suspected, follow definitive removal steps.
- Use fine‑pointed tweezers to grasp the tick as close to the skin as possible; pull upward with steady pressure.
- Clean the bite site with antiseptic; monitor for signs of infection or expanding lesions.
- Seek medical evaluation if redness spreads, fever develops, or the tick remains embedded despite removal attempts.
Complications and Long-Term Effects
Secondary Infections
Bacterial Superinfections
Subcutaneous ticks embed their mouthparts beneath the epidermis, creating a narrow tunnel that can remain unnoticed for days. The breach in the skin barrier provides a conduit for bacteria from the tick’s oral cavity, the surrounding environment, or the host’s own flora to colonize the wound. When bacterial proliferation exceeds the local immune response, a superinfection develops on top of the tick‑induced lesion.
The entry of bacteria occurs during the tick’s feeding process, when saliva containing antimicrobial proteins is injected to facilitate blood uptake. This saliva suppresses local immunity, allowing opportunistic microbes to establish infection. Additionally, the prolonged presence of the tick increases the duration of tissue exposure, raising the probability that skin‑resident organisms such as Staphylococcus or Streptococcus species will infiltrate the site.
Common bacterial agents associated with tick‑related superinfections include:
- Staphylococcus aureus (including methicillin‑resistant strains)
- Streptococcus pyogenes
- Borrelia burgdorferi (when co‑transmitted)
- Rickettsia rickettsii (in regions where spotted fever is endemic)
- Aeromonas spp. (in humid environments)
Clinical indicators of bacterial superinfection comprise increasing erythema, warmth, swelling, purulent discharge, and escalating pain at the bite location. Systemic signs such as fever, chills, or lymphadenopathy suggest spread beyond the local site. Prompt antimicrobial therapy, guided by culture results when available, is essential. Empiric coverage often starts with a broad‑spectrum agent targeting Gram‑positive organisms, followed by adjustment based on susceptibility patterns. Surgical debridement may be required for extensive necrosis or abscess formation.
Effective management combines removal of the tick, thorough cleaning of the wound, and vigilant monitoring for signs of bacterial overgrowth. Early intervention reduces the risk of complications such as cellulitis, septic arthritis, or systemic infection.
Fungal Co-infections
Subcutaneous tick attachment creates a portal for secondary microbial invasion. The wound environment—warm, moist, and rich in blood—supports opportunistic fungi that may colonize the bite site. Early fungal colonization can be indistinguishable from tick‑induced inflammation, delaying appropriate treatment.
Common fungal agents involved in co‑infection include:
- Dermatophytes (e.g., Trichophyton rubrum, Microsporum canis) – produce erythematous, scaly lesions that may extend beyond the tick bite.
- Yeasts (Candida spp.) – thrive in moist tissue, generating pustular or ulcerative changes.
- Molds (Aspergillus spp., Fusarium spp.) – cause necrotic plaques, especially in immunocompromised hosts.
Risk factors for fungal co‑infection are:
- Prolonged tick attachment (>24 h) increasing tissue damage.
- Inadequate wound cleaning or delayed removal of the tick.
- Underlying conditions such as diabetes, peripheral vascular disease, or immunosuppression.
- Environmental exposure to fungal spores (e.g., agricultural work, damp clothing).
Diagnostic approach combines clinical inspection with laboratory confirmation. Direct microscopy of exudate can reveal hyphal fragments or yeast cells, while culture on selective media identifies the species. Molecular techniques (PCR) provide rapid species‑level detection, guiding targeted antifungal therapy.
Management requires simultaneous eradication of the tick and the fungal pathogen. Recommended steps:
- Immediate mechanical removal of the tick using sterile forceps, avoiding compression of the mouthparts.
- Thorough debridement of the surrounding tissue to eliminate necrotic material.
- Empirical antifungal therapy pending culture results, typically an azole (e.g., itraconazole) for dermatophytes or a polyene (e.g., amphotericin B) for invasive molds.
- Adjust therapy based on susceptibility data; monitor for drug interactions, especially with concurrent anti‑tick antibiotics.
Prevention focuses on minimizing tick exposure, prompt removal, and proper wound care. Protective clothing, repellents, and regular skin examinations after outdoor activities reduce the likelihood of tick attachment and subsequent fungal colonization.
Dermatological Damage
Chronic Skin Changes
Subcutaneous ticks embed beneath the epidermis, creating a persistent nidus that provokes long‑term dermal alteration. The tick’s mouthparts remain attached to the dermal matrix, delivering saliva containing anticoagulants, anti‑inflammatory agents, and immunomodulators. Continuous exposure to these substances induces localized vascular dilation, fibroblast activation, and collagen remodeling, which manifest as chronic skin changes.
Typical chronic manifestations include:
- Firm, raised nodules persisting for weeks to months
- Hyperpigmented or hypopigmented halos surrounding the lesion
- Atrophic scarring after tick removal or spontaneous extrusion
- Persistent erythema or telangiectasia within the affected area
- Secondary hyperkeratosis due to chronic irritation
Histopathology frequently reveals a granulomatous infiltrate composed of macrophages, lymphocytes, and occasional eosinophils, with central necrosis surrounding residual tick parts. Fibroblastic proliferation leads to dense collagen bundles, producing the palpable nodule. Vascular changes result from prolonged vasodilatory mediator exposure, generating visible telangiectasia and erythema.
Differential diagnosis must exclude other causes of chronic dermal nodules, such as cysts, dermatofibromas, and cutaneous neoplasms. Accurate identification relies on patient history of outdoor exposure, localized tenderness, and the presence of a punctate entry scar. Imaging modalities—ultrasound or high‑frequency dermoscopy—can locate retained tick components, guiding surgical extraction.
Effective management involves complete removal of residual tick structures, followed by wound care to prevent secondary infection. Topical corticosteroids may reduce lingering inflammation, while silicone gel sheets can mitigate hypertrophic scarring. Regular follow‑up ensures resolution of chronic changes and identifies any delayed complications, such as secondary infection or atypical granuloma formation.
Scarring and Pigmentation
Subcutaneous tick attachment often leaves a permanent mark on the skin. The bite penetrates the epidermis, depositing saliva that can trigger a localized inflammatory response. As the wound heals, fibroblasts produce collagen, resulting in a raised or depressed scar. The scar’s texture depends on the depth of the lesion, the tick’s size, and the host’s healing capacity.
Pigmentation changes accompany the scar in many cases. Melanocytes may become hyperactive during inflammation, producing excess melanin that darkens the surrounding area. Conversely, loss of melanocytes can create hypopigmented patches. Both outcomes may persist for months or become permanent.
Factors influencing scar severity and pigment alteration include:
- Duration of attachment: longer feeding periods increase tissue disruption.
- Tick species: some species inject more potent anticoagulants and enzymes.
- Individual skin type: darker skin tones are more prone to post‑inflammatory hyperpigmentation.
- Prompt removal: early extraction reduces tissue damage and subsequent discoloration.
Treatment options focus on minimizing scar prominence and correcting pigment imbalance. Topical silicone gels, pressure therapy, and intralesional corticosteroids can flatten hypertrophic scars. For hyperpigmentation, hydroquinone, azelaic acid, or laser resurfacing are effective. Hypopigmented areas may respond to narrow‑band UVB phototherapy or microneedling combined with topical retinoids.
Monitoring the lesion for secondary infection or tick‑borne disease remains essential. Early medical evaluation ensures appropriate antimicrobial therapy and reduces the risk of complications that could exacerbate scarring and pigmentation disturbances.
Therapeutic Approaches
Topical Treatments
Acaricides
Subcutaneous ticks penetrate the epidermis and migrate into the dermal layer, where they remain attached to host tissue and feed on blood. The process begins when a tick, after questing on vegetation, contacts skin and inserts its hypostome. Salivary secretions containing anticoagulants and anesthetics facilitate painless insertion, allowing the parasite to move deeper until the surrounding inflammatory response encapsulates it in a granuloma. The resulting nodule often appears as a firm, slightly raised bump that may be tender or asymptomatic, depending on the tick species and duration of attachment.
Acaricides are the primary chemical agents used to eliminate ticks that have entered subcutaneous tissue. Their effectiveness relies on specific modes of action:
- Organophosphates (e.g., chlorpyrifos) inhibit acetylcholinesterase, leading to neurotoxicity in the tick.
- Pyrethroids (e.g., permethrin) disrupt voltage‑gated sodium channels, causing paralysis.
- Formamidines (e.g., amitraz) act on octopamine receptors, impairing locomotion and feeding.
- Isoxazolines (e.g., fluralaner) block GABA‑gated chloride channels, resulting in uncontrolled neuronal firing.
Application strategies differ according to the tick’s depth:
- Topical acaricide creams or gels penetrate the epidermis, reaching the dermal zone where the tick resides; concentration and vehicle viscosity are critical for adequate diffusion.
- Systemic acaricides administered orally or via injection distribute through the bloodstream, exposing the tick to lethal concentrations during blood meals.
- Injectable formulations combine a local anesthetic with an acaricide, allowing direct delivery into the nodule and immediate immobilization of the parasite.
Safety considerations include:
- Monitoring for hypersensitivity reactions at the application site.
- Avoiding excessive systemic exposure, particularly with organophosphates, due to potential neurotoxicity in the host.
- Observing withdrawal periods for systemic agents in patients with comorbidities or those receiving other pharmacotherapies.
Effective management of subdermal tick infestations therefore depends on prompt identification of the nodule, selection of an appropriate acaricide class, and adherence to dosage protocols that maximize tick mortality while minimizing host risk.
Symptomatic Relief
Subcutaneous ticks embed beneath the skin, often producing localized swelling, pain, and pruritus. Prompt relief of these symptoms reduces discomfort and limits secondary complications.
Analgesic and anti‑inflammatory agents address pain and edema. Oral non‑steroidal anti‑inflammatory drugs (NSAIDs) such as ibuprofen provide systemic relief, while topical NSAID gels target the affected area with minimal systemic exposure.
Antihistamines counteract histamine‑mediated itching. Second‑generation oral antihistamines (e.g., cetirizine, loratadine) reduce pruritus without causing sedation. Topical antihistamine creams may be applied for localized relief.
Cold therapy decreases vascular congestion and nerve activity. A clean, cold compress applied for 10–15 minutes, repeated every hour, alleviates swelling and tingling sensations.
Corticosteroids suppress inflammatory cascades. Short courses of oral prednisone or a single dose of a potent topical steroid (e.g., betamethasone) diminish erythema and tissue irritation when symptoms are pronounced.
If secondary infection is suspected—characterized by increasing redness, warmth, or purulent discharge—empirical antibiotic therapy should be initiated. Oral doxycycline or amoxicillin‑clavulanate cover common skin pathogens and tick‑borne bacteria.
Wound care maintains a clean environment for tick removal and healing. After extraction, cleanse the site with antiseptic solution, apply a sterile dressing, and monitor for signs of infection over 48–72 hours.
Symptomatic relief protocol
- NSAID (oral or topical) for pain and swelling
- Second‑generation antihistamine for itching
- Cold compress, 10–15 min, repeat hourly
- Short‑term corticosteroid if inflammation severe
- Antibiotic if infection indicators present
- Antiseptic cleaning and sterile dressing post‑removal
Adhering to this regimen mitigates discomfort, prevents escalation, and supports recovery after a subcutaneous tick embeds in the skin.
Systemic Medications
Oral Therapies
Subcutaneous ticks embed beneath the skin after initial attachment, creating a raised, often painful nodule that may be mistaken for a cyst or abscess. The parasite’s mouthparts remain anchored while the body enlarges, prompting local inflammation and occasional secondary bacterial infection.
Oral medications constitute the primary pharmacologic approach for managing these infestations. Systemic agents reach the parasite through the bloodstream, eliminating the tick and addressing associated complications.
- Ivermectin – single dose of 200 µg/kg; effective against active ticks and reduces inflammation.
- Doxycycline – 100 mg twice daily for 7–10 days; treats potential tick‑borne bacterial pathogens and provides anti‑inflammatory benefits.
- Azithromycin – 500 mg once daily for 3 days; alternative for patients intolerant to tetracyclines, targeting bacterial co‑infections.
- Mebendazole – 100 mg twice daily for 3 days; off‑label use for tick eradication when contraindications exist for first‑line agents.
- Analgesic/anti‑inflammatory agents – ibuprofen 400 mg every 6 hours as needed; alleviates pain and swelling.
Oral therapy should be initiated promptly after clinical identification of the subdermal lesion. Follow‑up examination confirms tick removal, monitors for residual inflammation, and assesses the need for additional antimicrobial coverage.
Adjunctive Treatments
Adjunctive therapies complement primary removal of embedded ticks and reduce tissue reaction, infection risk, and symptom persistence. Systemic antibiotics, typically doxycycline 100 mg twice daily for 10–14 days, target possible Borrelia or Rickettsia transmission and are indicated when fever, rash, or laboratory evidence of infection appears. Antihistamines such as cetirizine 10 mg daily alleviate pruritus and local swelling, while short courses of oral corticosteroids (e.g., prednisone 20 mg daily for 5 days) suppress severe inflammatory responses that may follow tick extrusion.
Topical agents support wound healing and prevent secondary bacterial colonization. Mupirocin ointment applied twice daily for 7 days provides coverage against Staphylococcus aureus and Streptococcus pyogenes. Moisture‑retaining dressings containing hydrogel reduce necrotic tissue desiccation and promote granulation. For patients with persistent pain, non‑steroidal anti‑inflammatory drugs (ibuprofen 400 mg every 6 hours) offer analgesic and anti‑edematous effects.
Adjunctive measures also include patient education on tick‑avoidance practices, thorough skin inspection after outdoor exposure, and prompt reporting of atypical lesions. Regular follow‑up appointments enable early detection of delayed complications such as granuloma formation or serologic conversion. Implementing these supportive interventions alongside definitive tick extraction improves overall outcomes and minimizes long‑term sequelae.
Public Health Implications
Epidemiology
Global Distribution
Subcutaneous tick infestations in humans are reported across temperate and subtropical zones where ixodid ticks thrive. Surveillance data indicate that cases concentrate in regions with high outdoor activity and dense wildlife reservoirs, but occasional reports emerge from urban settings where pets introduce tick species.
- North America: Dermacentor variabilis and Ixodes scapularis identified in the United States and southern Canada; most cases occur in the Midwest and Northeastern United States.
- Europe: Dermacentor reticulatus and Ixodes ricinus documented in Central and Eastern Europe, with notable incidence in Germany, Poland, and the Baltic states.
- Asia: Haemaphysalis longicornis and Ixodes persulcatus recorded in Japan, Korea, China, and Siberian Russia; sporadic cases reported from South‑East Asian highlands.
- South America: Amblyomma cajennense and Rhipicephalus sanguineus detected in Brazil, Argentina, and Colombia; cases linked to rural agricultural work.
- Africa: Rhipicephalus appendiculatus and Amblyomma variegatum observed in sub‑Saharan regions, especially Kenya and South Africa; isolated reports from North African Mediterranean coasts.
- Oceania: Ixodes holocyclus identified in eastern Australia; limited incidents in New Zealand involving Haemaphysalis longicornis.
Incidence correlates with climatic suitability for tick development, host abundance, and human exposure patterns. Climate change expands the geographic range of several species, prompting emergence of subcutaneous infestations in previously unaffected latitudes.
Outbreak Management
Subcutaneous tick infestations present a public‑health challenge that requires systematic outbreak management. Prompt identification of cases enables health authorities to assess the scope of exposure and to initiate containment measures before further transmission occurs.
Effective response relies on coordinated reporting, rapid laboratory confirmation, and immediate communication with medical facilities. Standardized case definitions ensure consistency across jurisdictions, while digital surveillance platforms collect real‑time data on incident clusters.
Key actions in managing an outbreak include:
- Immediate notification of local health departments upon detection of a subcutaneous tick case.
- Verification of tick species and infection status through accredited laboratories.
- Isolation of affected individuals for clinical assessment and treatment.
- Dissemination of targeted public advisories outlining preventive behaviors, such as proper clothing, tick checks, and prompt removal techniques.
- Deployment of environmental control measures in identified hotspots, including vegetation management and acaricide application.
- Ongoing monitoring of treatment outcomes and post‑exposure follow‑up to detect secondary complications.
Continuous evaluation of intervention effectiveness guides resource allocation and informs policy adjustments, ensuring that future occurrences are mitigated with minimal impact on community health.
Educational Initiatives
Awareness Campaigns
Effective public‑health initiatives targeting hidden tick infestations rely on precise messaging, measurable outreach, and coordinated delivery. Campaigns must convey the biological pathway by which ticks embed beneath the skin, the early visual cues—small, raised lesions, localized itching, or unexplained swelling—and the steps required for safe removal and medical assessment.
Key elements of a successful awareness drive include:
- Clear definition of at‑risk groups (outdoor workers, hikers, pet owners) and geographic hotspots.
- Concise visual aids illustrating tick entry points, typical incubation periods, and distinguishing features of subcutaneous lesions.
- Instructional content on self‑examination techniques, proper removal tools, and when to seek professional care.
- Distribution channels such as community workshops, social‑media micro‑videos, healthcare‑provider handouts, and signage in parks and recreation areas.
- Monitoring framework that tracks outreach reach, knowledge retention through brief quizzes, and reported cases of early detection.
Implementation should integrate local health departments, wildlife agencies, and educational institutions to ensure consistent terminology and avoid misinformation. Periodic evaluation—comparing baseline awareness levels with post‑campaign surveys—identifies gaps and informs iterative refinement.
Sustained engagement, reinforced by seasonal reminders aligned with peak tick activity, maximizes community vigilance and reduces the incidence of concealed tick colonization.
Prevention Strategies
Subcutaneous tick infestations develop when ticks attach to the skin, penetrate the epidermis, and lodge within the dermal tissue. Preventing this deep attachment requires eliminating exposure, reducing skin contact with tick habitats, and promptly removing any attached arthropods before they embed.
Effective prevention measures include:
- Wearing long sleeves and trousers treated with permethrin when entering wooded or grassy areas.
- Applying EPA‑registered repellents containing DEET, picaridin, or IR3535 to exposed skin and clothing.
- Conducting thorough body inspections after outdoor activities, focusing on hidden regions such as the scalp, armpits, and groin.
- Showering within two hours of returning from a tick‑risk environment to dislodge unattached specimens.
- Maintaining landscaping by trimming vegetation, removing leaf litter, and creating a barrier of wood chips or gravel around residential structures.
- Keeping pets on regular veterinary tick‑preventive programs to reduce the likelihood of animal‑borne transfer to humans.
Adhering to these practices minimizes the probability of ticks reaching subdermal layers and reduces associated health risks.